Senior Unit Operations Laboratory

What you'll get out of our senior laboratory experience
Leadership
Teamwork
Hands On Learning
Communication
Technical Content
Time Management
Absorption
Packed column filled with ceramic Raschig rings for the purification of gas mixtures using a liquid absorbent. Air/ammonia gas mixtures are stripped of ammonia using water at varying flow rates. Students apply concepts of mass transfer and unit operations to calculate key column parameters and mass transfer coefficients from experimental data. Understanding the effect of operating variables on mass transfer helps chemical engineers design effective and efficient separation equipment.

Distillation
Pilot-scale distillation column with dedicated control room featuring industrial equipment, instrumentation, and controls. Working in teams, students experience a realistic industrial environment, purifying a continuous stream of 20 w% methanol mixed with water and obtaining >99w% pure methanol. Experimenting with pilot-scale equipment helps chemical engineers validate and understand the effect of operating conditions on process results at levels that could scale up to industrial production capacity.

Pressure-drop in Pipes
Measurement of pressure loss of water as it travels through pipes of different materials and diameters. Applying knowledge of fluid mechanics, students determine the friction factor (f) for the various pipes at multiple flow conditions. Friction factors are used by chemical engineers to predict pressure losses in pipes to correctly size pumps for liquid transfer service.
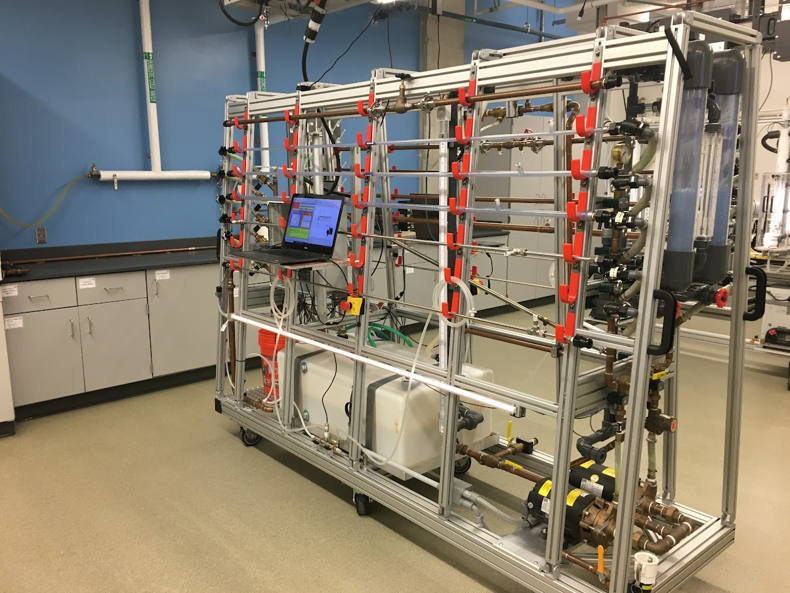

Process Control
Flexible multi-tank system equipped with automatic control valves, pumps, and digital instruments. Students design and implement control strategies to measure and regulate desired operating variables (flow, level, temperature, and pH). Applying concepts of process dynamics and control enables chemical engineers to specify controller settings that successfully maintain a process at target operating conditions.
Reaction Kinetics
Experimental measurements of rates at which reactants convert into products (A + B à C + D). Using the concepts of chemical reaction engineering, students determine the parameters for kinetic equations that help engineers predict reaction rates and design industrial-scale reactors.

Vapor-Liquid Equilibrium
This equipment provides high-accuracy measurements of boiling points of pure solvents and two-component mixtures (less than 0.2% error). Students combine experimental data with principles of thermodynamics to construct temperature-composition plots known as Txy diagrams. The ability to calculate these Txy plots is key for chemical engineers to design separation equipment such as distillation columns.



